Will humans be able to build on the Moon and Mars? Thanks to Washington State University researchers, the sci-fi fantasy is moving closer to reality. Scientists discovered powerful liquid solvents capable of extracting vital materials from lunar and Martian rock dust for 3D printing. These findings are crucial for long-term space exploration.
The study employed advanced machine learning and computational modeling techniques. Researchers focused on a type of powerful solvent known as ionic liquids. These substances are essentially salts in a liquid state, which hold promise for space mining. They’re great for dissolving things without evaporating (low vapor pressure) and can conduct electricity well (high electrochemical stability). The study looks at using these ionic liquids to dissolve and extract metals from the Moon and Mars soil.

When metals from the soil dissolve in ionic liquids, they form what’s known as “solvation shells.” Picture a metal ion (like sodium, magnesium, or aluminum) being surrounded by molecules of the ionic liquid, like a protective bubble. This shell is crucial for the metal to dissolve effectively.
The team employed machine learning to sift through hundreds of thousands of potential ionic liquid candidates, seeking those capable of digesting lunar and Martian materials and extracting essential elements like aluminum, magnesium, and iron. These solvents would ideally regenerate themselves and possibly yield oxygen or water as byproducts.
“The machine learning work brought us down from the 20,000-foot to the 1,000-foot level,” study author Soumik Banerjee, associate professor in WSU’s School of Mechanical and Materials Engineering, in a media release. “We were able to down select a lot of ionic liquids very quickly, and then we could also scientifically understand the most important factors that determine whether a solvent is able to dissolve the material or not.”
This research is a part of NASA’s Artemis mission, which aims to send humans back to the Moon and eventually to Mars and beyond. A critical aspect of these long-term missions is in situ resource utilization — using the materials available in space to manufacture necessary structures, tools, or parts.
“In situ resource utilization is a big deal over the next couple of decades for NASA,” notes Banerjee. “Otherwise, we would need a terribly high payload of materials to carry from Earth.”
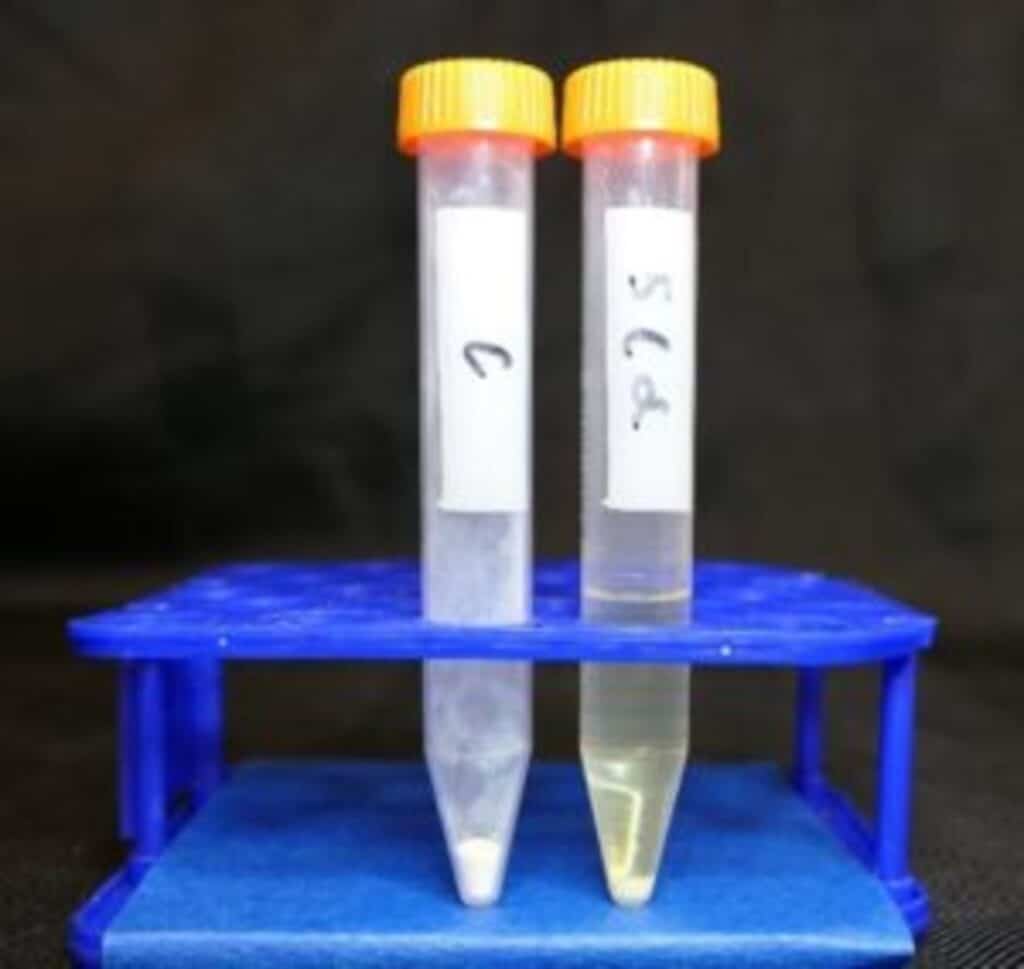
One of the challenges in mining these extraterrestrial materials is the need for environmentally friendly and energy-efficient methods that do not rely on water, as it is scarce on the Moon. Ionic liquids, which have been studied by Banerjee’s group for over a decade for battery applications, could now be the solution.
Researchers have now identified about half a dozen strong candidates, focusing on key factors such as the size of the molecular ions, the surface charge density, and the ions’ mobility within the liquids. Collaborating with the University of Colorado, they have tested a few ionic liquids in the lab for their ability to dissolve compounds.
They found that the “tightness” or compactness of the solvation shell depends on the charge of the metal ion and the properties of the ionic liquid. Also, they observed that some energy barriers increase as the metal ion’s charge increases. This barrier affects how long the metal stays in its solvation shell.

The next step is to build a lab-scale or pilot-scale reactor to test these solvents with materials similar to lunar regolith. Understanding these interactions helps in selecting the best ionic liquids for extracting metals from lunar and Martian soil. This knowledge is important for future space missions where we might want to extract and use these metals for construction, manufacturing, or other purposes on the Moon or Mars.
This innovative research opens the door to more sustainable and efficient resource utilization in space, marking a significant step forward in the field of space exploration and the potential for human colonization.
The study is published in the Journal of Physical Chemistry B.










GO COUG$ !!!!!!!
No one’s going to mars
We can’t even figure out how the Egyptians stacked a pile of rocks
Why would anyone in their right mind want to live on Mars or the moon? Seriously. It would be an absolutely miserable existence. Even if our idiot leaders bad decisions caused the earth to become a radioactive wasteland, life would still be easier here.
Extracting metals from regolith would also enable building large spacecraft in space station “shipyards” located at Lagrange points, spacecraft whose designs do not rely on being aerodynamic. Much simpler and more efficient.