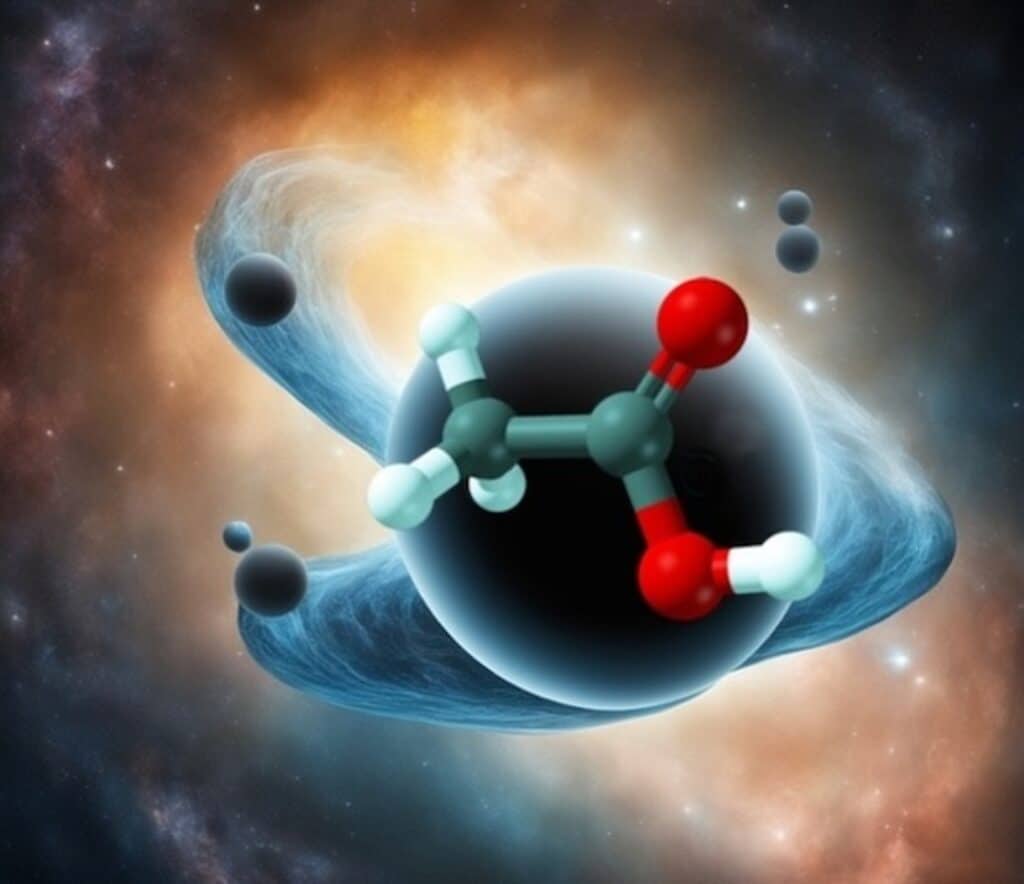
When you think of the ultimate information scramblers in the universe, black holes likely come to mind. These cosmic behemoths are known for their ability to completely jumble up information, down to the quantum level, as quickly and thoroughly as the laws of physics allow. But a new study suggests that molecules, despite their minuscule size, can be just as formidable at scrambling quantum information as their astronomical counterparts.
Researchers from Rice University and the University of Illinois Urbana-Champaign have shown that chemical reactions can scramble quantum information nearly as efficiently as black holes. The findings, published in the Proceedings of the National Academy of Sciences, shed new light on a long-standing problem in chemical physics.
“This study addresses a long-standing problem in chemical physics, which has to do with the question of how fast quantum information gets scrambled in molecules,” says study author Peter Wolynes, a theorist from Rice University, in a media release. “When people think about a reaction where two molecules come together, they think the atoms only perform a single motion where a bond is made or a bond is broken.
“But from the quantum mechanical point of view, even a very small molecule is a very complicated system. Much like the orbits in the solar system, a molecule has a huge number of possible styles of motion — things we call quantum states. When a chemical reaction takes place, quantum information about the quantum states of the reactants becomes scrambled, and we want to know how information scrambling affects the reaction rate.”
To tackle this question, researchers borrowed a mathematical tool from black hole physics known as out-of-time-order correlators, or OTOCs. These correlators measure how much tweaking one part of a quantum system at a given instant will affect the motions of the other parts, providing insight into the speed and effectiveness of information spread throughout the molecule. In essence, OTOCs are the quantum equivalent of Lyapunov exponents, which measure unpredictability in classical chaotic systems.
“How quickly an OTOC increases with time tells you how quickly information is being scrambled in the quantum system, meaning how many more random looking states are getting accessed,” explains study co-author Martin Gruebele, a chemist at Illinois Urbana-Champaign who is a part of the joint Rice-Illinois Center for Adapting Flaws as Features funded by the National Science Foundation. “Chemists are very conflicted about scrambling in chemical reactions, because scrambling is necessary to get to the reaction goal, but it also messes up your control over the reaction.”
The calculation of OTOCs revealed that chemical reactions with a low activation energy at low temperatures, where quantum tunneling dominates, can scramble information at nearly the quantum limit, rivaling the efficiency of black holes. Quantum tunneling is a phenomenon where particles can pass through an energy barrier even if they don’t possess sufficient energy to overcome it classically.
However, when the simple chemical reaction model was embedded in a larger system, such as a large molecule’s own vibrations or a solvent, the chaotic motion and information scrambling were suppressed.
“In a separate study, we found that large environments tend to make things more regular and suppress the effects that we’re talking about,” says study co-author Nancy Makri, a chemist at Illinois Urbana-Champaign. “So we calculated the OTOC for a tunneling system interacting with a large environment, and what we saw was that the scrambling was quenched — a big change in the behavior.”
The findings have practical implications in various fields. For instance, they can help set limits on how tunneling systems can be used to build qubits, the building blocks of quantum computers. To improve the reliability of these advanced machines, information scrambling between interacting tunneling systems needs to be minimized. The insights gained from this study could also be relevant for light-driven reactions and the design of advanced materials.
“There’s potential for extending these ideas to processes where you wouldn’t just be tunneling in one particular reaction, but where you’d have multiple tunneling steps, because that’s what’s involved in, for example, electron conduction in a lot of the new soft quantum materials like perovskites that are being used to make solar cells and things like that,” notes Gruebele.
By combining mathematical tools from black hole physics and chemical physics, this groundbreaking research has not only shed light on the fundamental nature of quantum information scrambling in molecules but also opened up new avenues for practical applications. As our understanding of these complex quantum processes deepens, we may be able to harness this knowledge to develop more efficient and reliable quantum technologies, from quantum computers to advanced materials.











Comments